Registration now open!

About the Conference
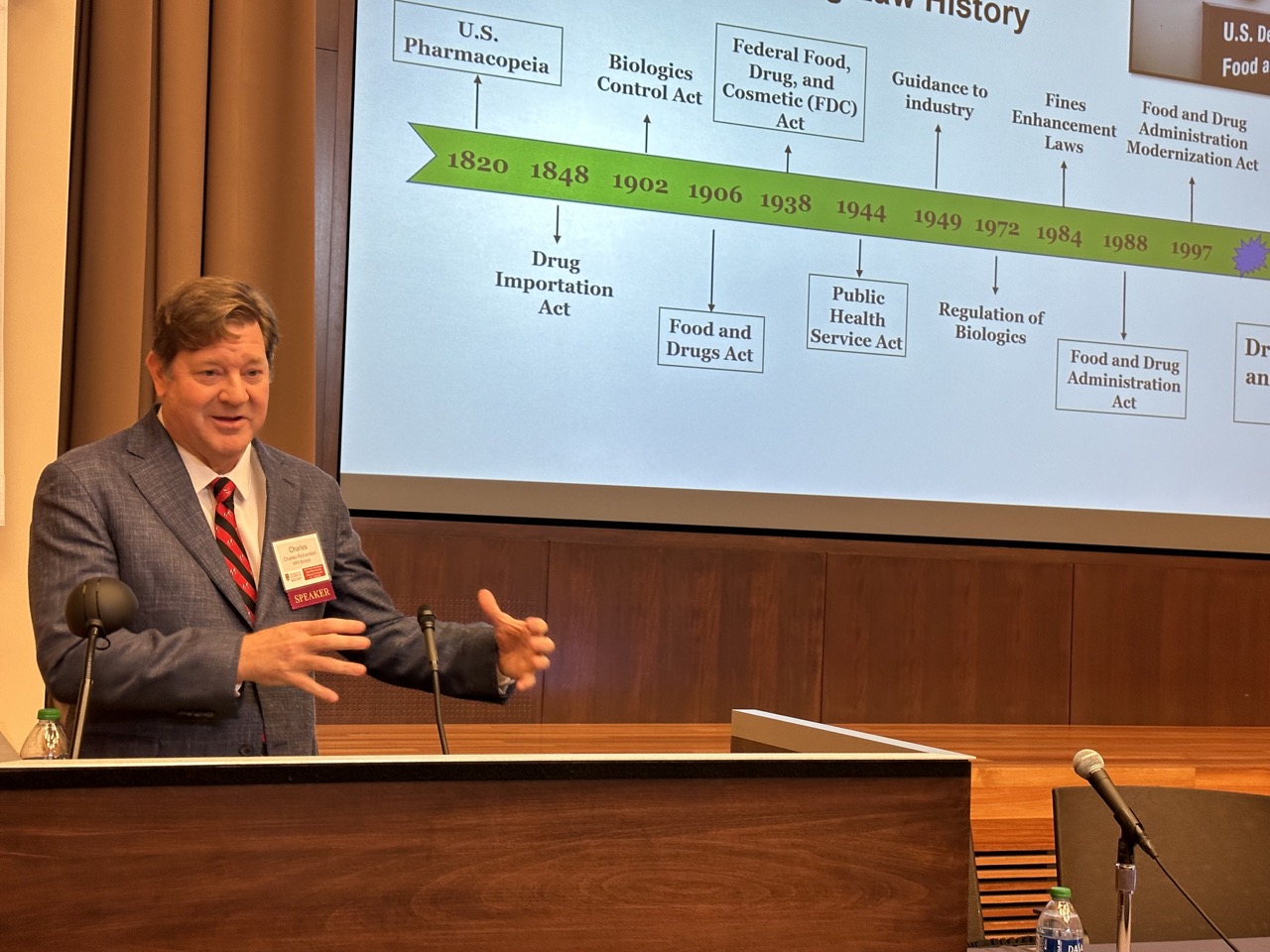
The International GMP Conference has been co-sponsored by the University of Georgia College of Pharmacy and U.S. Food & Drug Administration (FDA) since 1976. It is a not-for-profit conference that was founded on the principle that FDA and the pharmaceutical industry needed a neutral forum in which to discuss and debate critical drug and biologic manufacturing issues. It has evolved over the past four decades into a forum where very complex and sophisticated policies and procedures are presented and discussed.
The speakers are distinguished leaders in FDA, foreign governments and industry who will present the latest information on issues related to pharmaceutical quality and manufacturing. Attendees will leave the conference with valuable information and ideas that will facilitate the production of safe, high quality drugs for consumers. The conference is the oldest and best GMP conference and is consistently rated outstanding by its attendees.